

Our media are produced under GMP conditions as well as EMA and FDA regulations. The media are available in liquid or in powder formulation.

Our culture media are completely synthetic.
They are free of
• Serum
• Animal components
• Hydrolysate
• Growth factor

Medium for production of virus and recombiant proteins. Serum-free, animal component-free, hydrolysate-free and fully defined.
Medium for viral vector replication and transient expression. Serum-free, animal component-free, hyrdolysate-free and fully defined.

Medium for virus production. Serum-free, animal component-free, hydrolysate-free and fully defined.
Medium for clonal growth of single cells. Serum-free, animal component-free, hydrolysate-free and fully defined.
Medium for clonal growth of single cells. Serum-free, animal component-free, hydrolysate-free and fully defined.

Medium for production of virus and recombiant proteins. Serum-free, animal component-free, hydrolysate-free and fully defined.

Medium for production of virus and recombiant proteins. Serum-free, animal component-free, hydrolysate-free and fully defined.

Medium for production of virus and recombiant proteins. Serum-free, animal component-free, hydrolysate-free and fully defined.

Medium for virus production. Serum-free, animal component-free, hydrolysate-free and fully defined. (Licensed-out)
Medium for recombinant protein production. Serum-free, animal component-free, hydrolysate-free, fully defined.

Medium for recombinant protein production. Serum-free, animal component-free, hydrolysate-free, fully defined.

Medium for recombinant protein production. Serum-free, animal component-free, hydrolysate-free, fully defined.

Platforms, more than their single modules

Punctual solutions seldom bring sustainable value to clients, Therefore we have an integral approach. We have developed platform technologies for the manufacturing of recombiant proteins and vaccines. Our platforms consist of host cell lines, culture media and cell cultivation methodology from cell thaw up to harvest of a production bioeactor.
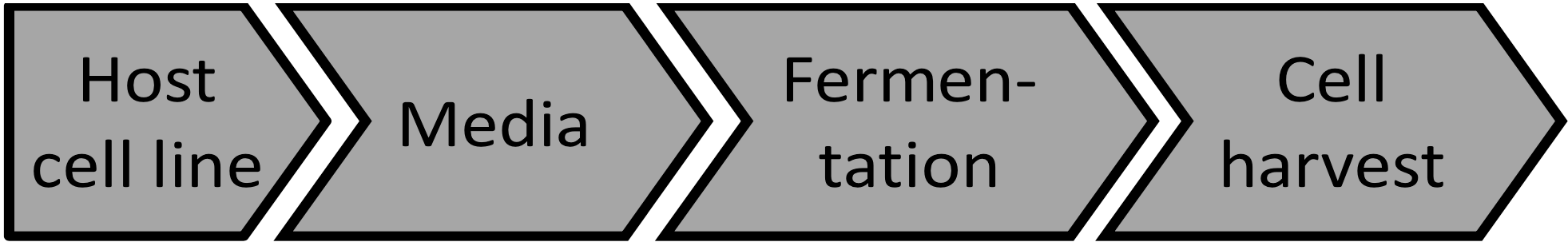
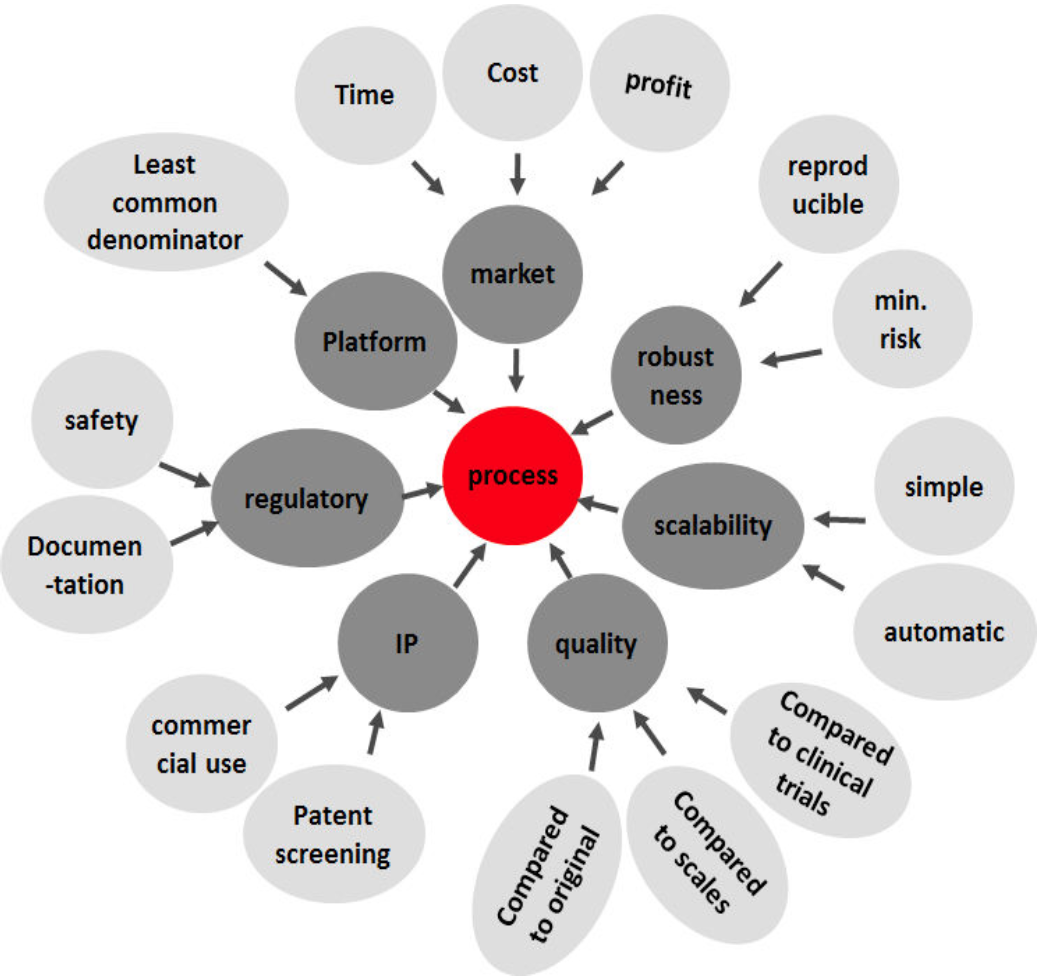
Using platforms we avoid repetitive development of culture media or bioreactor processes. Our platforms offer a holistic approach to our clients with the goal of reaching he market quickly. Our platforms consider all aspects of manufacturing while developing the process such as IP, regulation, scalability and robustness.

Our platforms at a glance

ProteinMax-
platform for protein production

We have a large platform for rapid production
of target proteins on a large scale with the desired quality. The protein
platform is a combination of multiple other platforms.
The protein platform consists of the following subplatforms:
1. Cell line generation platform consisting of vector, host line and the
clone selection process.
2. Culture media platform consisting of the single cell cloning medium,
the basal medium and the feed media.
3. Our GlycoShift platform consisting of nutrient cocktails for altering
the glycosylation.
4. The bioreactor platform made up of scaled down models of larger bioreactors
for fed-batch processes including cell harvesting.

The platform consists of our proprietary
expression vector, the CHO DG44 host cell line and a clone
selection process.
We offer cell line generation to our clients as a service.
Multiple cell lines are generated as long as all of them are expressing
biosimilarities. Our focus in cell line generation is the biosimilarity.
Therefore we use our platform known as GlycoShift during the cell line
generation.

We have multiple platforms for producing any
vaccine rapidly with high reproducibility and scalability.
Our platforms have been proven in the production of vaccines against Covid
-19, foot and mouth diseases, rabies, AAV as well as LV
and AV.
Virus and recombinant protein production with HEK293 cells.
Currently in use for 2 different vaccine projects against SARS-CoV-2.
The platform contains suspension adapted HEK293 cells. Animal component free and cultivated in a high titer fed-batch process and cell harvest procedure.
Learn moreViral vector production and transient protein expression with HEK293 cells.
This platform is used in vaccine development against Covid-19.
The platform contains suspension adapted HEK293 cells. Animal component free, transfection medium and transient expression protocol.
Learn moreVirus production with adherent VERO cells.
Currently in use for 2 different vaccine projects against SARS-CoV-2.
The platform contains adherent growing VERO cells. They are animal component free utilising a microcarrier based bioreactor process.
Learn moreVirus production with suspension adapted BHK-21 cells.
Used in viral production of Foot/Mouth disease and rabies across 3 continents.
The platform contains suspension adapted BHK-21 cells. They are animal component free and stirred in our bioreactor tank process.
Learn more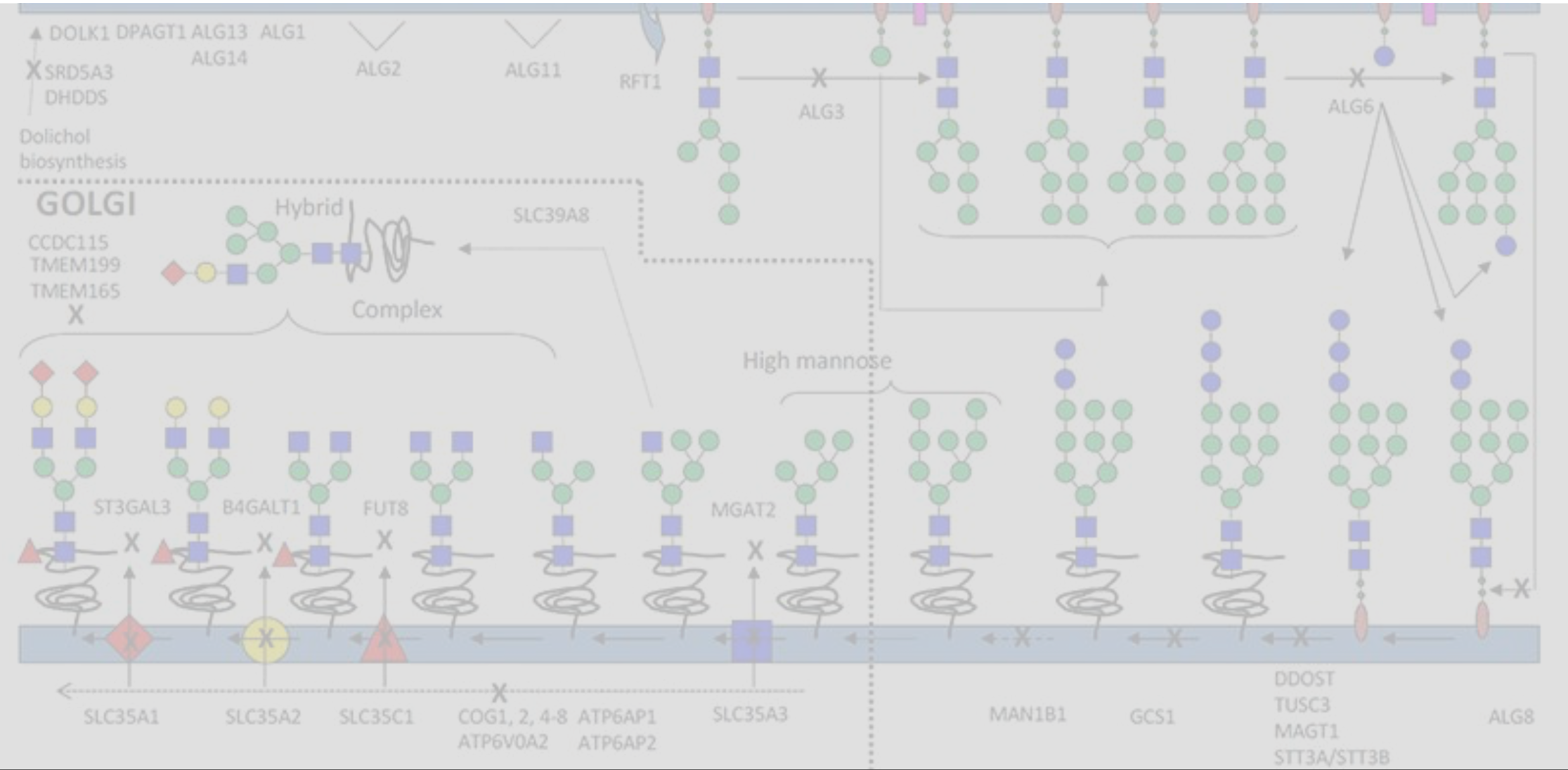
We have identified cellular nutrients that shift the secondary metabolism of cells in the desired direction. That is why we shift the glycolytic pathway of the cells in the wanted direction.
Without genetic interference
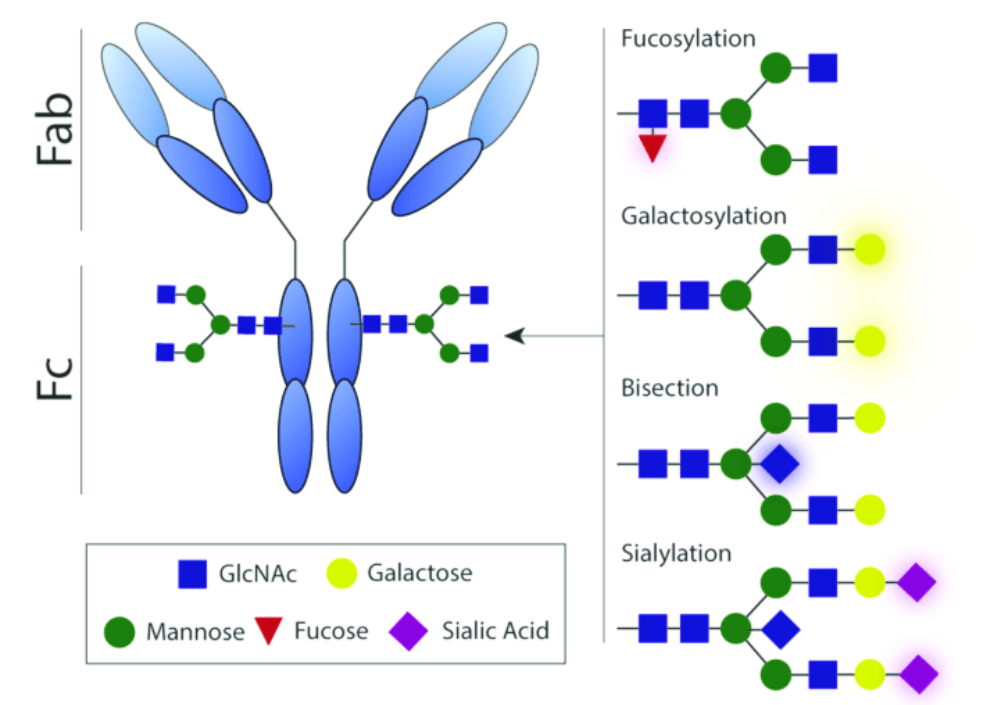
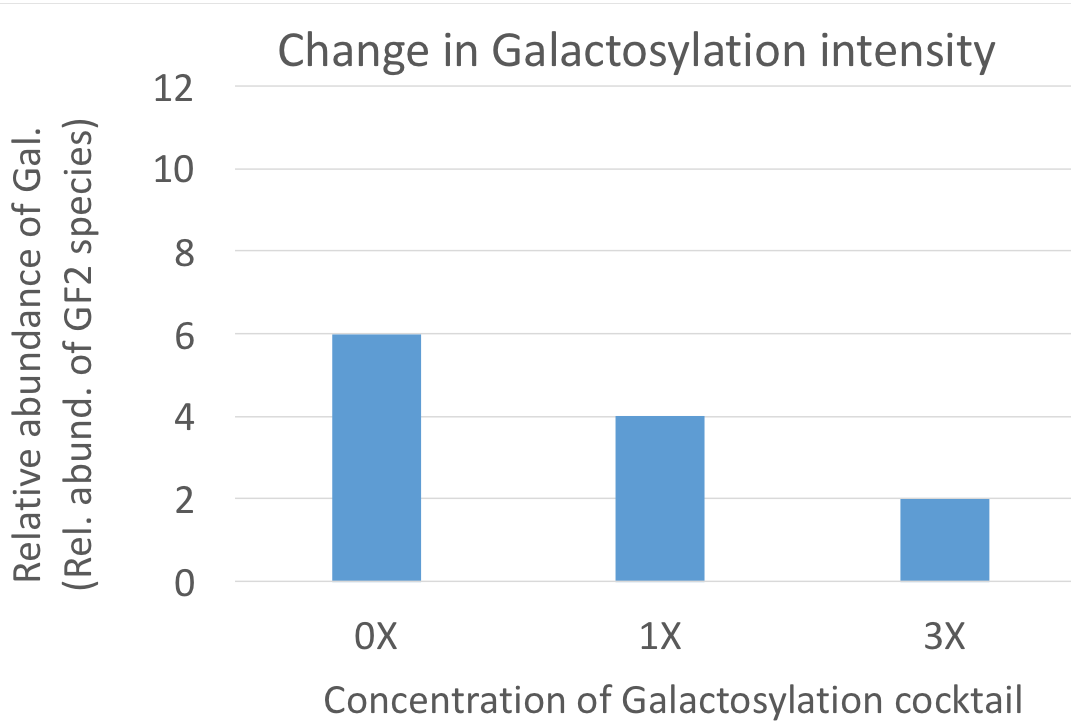
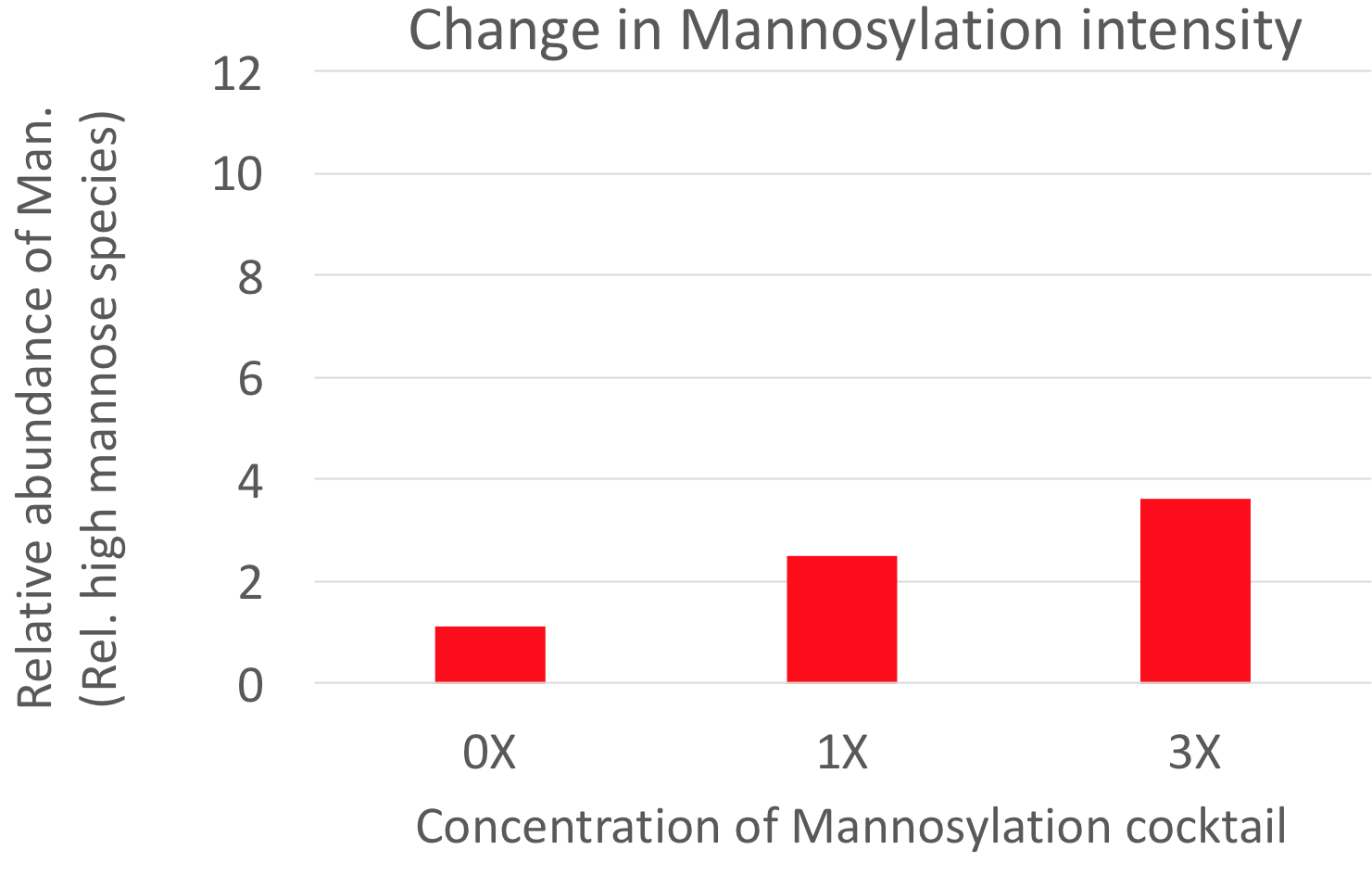
We shift protein galactosylation or mannosylation in the desired direction. We achieve this without genetic interference within the cells. There is no need to express exogenous glycosylation enzymes in the cells. Thus, without altering the master cell bank we can obtain the desired glycosylation profile.
Our specialised nutrient cocktails get formulated
into novel cell culture media. We utilize these media to modify protein
quality attributes.
These kinds of culture media are specifically designed to alter protein
mannosylation or galacosylation.

Our modularly assembled platform enables us to be among the first to develop novel culture media. We utilize a toolbox taking into consideration the metabolism of the specified cell type, for which to develop our products. The resulting media can be produced in a record time while surpassing other alternatives on the market.

Our in depth understanding of cellular metabolic
pathways enable us to develop media recipes, that correspond to the metabolic
needs of the particular cell type.
Our media recipes are not only optimised for cell growth but also optimised for
an efficient metabolism.
In all of our media the cells generate more cellular energy and less metabolic
waste products. Across all used cells the acidic waste is reduced enabling the
pH in the bioreactor to remain constant. This benefit simplifies cell cultivation
on a large scale.

All of our culture media are products of our
NutriBalance platform.
NutriBalance is built modularly, thus media development is simplified.
Most importantly media development for a novel cell line doesn´t take a lot of
time for us anymore. We combine modules by taking the specific cell metabolism
into account with an algorithm. This enables us to find the appropriate
culture medium for the cell line in a record time.

Our NutriBalance media platform is universally applicable to develop
any novel culture medium. It is applicable for not only animal cells
but also stem cells, bacteria as well as yeast.
We have also developed novel media for unusual cell lines like
Vero suspension cells, MDCK suspensison cells and MRC-5 suspension cells.

The cell science company

We are a life science company with proprietary technology, know-how
and experience in manufacturing therapeutic proteins. We accomplish this in the shortest time with the
most economic measures.
We have developed platform technologies for vaccine and recombinant protein production.
We provide our clients with production cells, culture media and our service to optimize
protein glycosylation.

Dr. Cayli started his venue into cell culture at GFB (Gesellschaft für biotechnologische Forschung) in Germany as a PhD student. His industry experience began at Roche Diagnostics in Penzberg, Germany where he transferred production processes from the US to Germany. Dr Cayli later worked at Boehringer Ingelheim in Biberach, Germany as head of the cell culture technology group. During this time he designed production processes to run on a 15.000 L scale. He later founded Cellca GmbH in Germany and developed one of the most powerful platform technologies in the world for cell line generation and media design.

Mr. Erdem is the former head of bioprocess division sales at Sartorius Turkey. He started his career as a management trainee at Sartorius Turkey. During his 16 year career at Sartorius Turkey Mr. Erdem worked as a project manager, sales manager as well as director of sales. Thanks to his vast experience in sales and marketing in the industry Mr. Erdem co-founded Florabio as well as Florabio Academy with Dr. Cayli and Dr. Walter.

Florabio AS
Gülbahce Mah. Gülbahce Cad.
Teknopark Izmir No: 01/43/04
35430 Urla/Izmir-TURKEY
info@florabio.com.tr
Phone: +90 232 502 3321